What Is the Halogen in Period 5
Fluorine chlorine bromine iodine and astatine definitely are halogens. So our answer is clearly iodine iodine is the one which lie in period five.
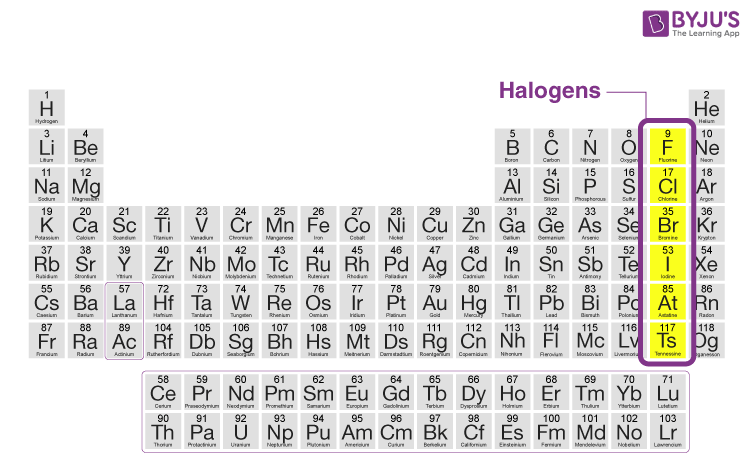
Halogens Definition Uses Compounds Properties Of Halogens
Is the halogen in the period 3.
. The halogen elements are fluorine chlorine bromine iodine astatine and possibly tennessine. The energy is then passed th. R00821 latmmolk 308 atm 12 atm 023 atm 401 atm 494 atm.
The word halogen means salthalo- makersgengeneratorsThe anionshalidesof the halogen family are part of a variety of salts which are ionic compounds. The iodine element in period 5 of the periodic table is a halogen. Iodine tends to be a little bit expensive.
As per Aufbau principle electrons of an element fill the energy levels in an increasing order. 1 Show answers Another question on Chemistry. What effect does cobalt have on the body.
Question Bank Solutions 7377. Depending on who you ask there are either 5 or 6 halogens. QUESTION 10 What is the name of the halogen in Period 5.
Answer 1 of 3. Br Xe Te I N. It is an alkali metal the most reactive group in the periodic table having properties and similarities with both other alkali metals and other period 5 elements.
Select the element that is a halogen in Period 5. A hydrogen mononitrogen dioxide b. We usually use the X symbol for substitution of halogen atoms.
It can cause heart muscle disease toxic cardiomyopathy after too much exposure. New questions in Science. Chemistry questions and answers.
The vertical columns are the Groups the horizontal rows are the. For halogenation of olefins we would thus write H_2CCH_2 X_2 rarr XCH_2-CH_2X In general XCl Br. The halogens are placed in Group 7 of the Perodic Table.
They are rubidium Rb strontium Sr yttrium Y zirconium Zr niobium Nb molybdenum Mo technetium Tc ruthenium Ru rhodium. Group 17 occupies the second column from the right in the periodic table and contains fluorine F chlorine Cl bromine Br iodine I astatine At and tennessine Ts. The artificially created element 117 tennessine Ts may also be a halogen.
The halogen elements are the six elements in Group 17 of the periodic table. Maharashtra State Board SSC Marathi Semi-English 10th Standard इयतत १० व Question Papers 172. The halogens ˈhælədʒən ˈheɪ - - loʊ - - ˌdʒɛn are a group in the periodic table consisting of five or six chemically related elements.
Radon strontium QUESTION 11 What is the name of the compound with the formula HNO2. QUESTION 10 What is the name of the halogen in Period 5. A halogen from period 4.
According to question we have to tell which halogen will lie in period five. What is halogen in period 4 on the periodic table. Noun any of the five elements fluorine chlorine bromine iodine and astatine that form part of group VIIA of the periodic table and exist in the free state normally as diatomic molecules.
What is the pressure of 0500 moles of carbon dioxide gas in a 25 l tank and at a temperature of 301 k. Fluorine F chlorine Cl bromine Br iodine I and astatine At. An increase in red blood cells polycythemia may be a symptom of too much cobalt.
Which element is a metal in period 5 of the periodic table. What is a halogen in period 5. Chlorine is a halogen in group 17 and period 3.
Energy usually enters ecosystems as 1_____and is captured in chemical form by photosynthesizer like 2______and algae. Astatine and tennessine are radioactive elements with very short half-lives and thus do not occur naturally. Its symbol would be I.
This is the next-to-last column of elements on the righthand side of the table. Iodine element number 53 is the halogen Group 17 element in period 5. Chemistry 22062019 1140.
The general electronic configuration of group 17 elements is ns 2 np 5. Cobalt is toxic to the heart muscle. What element is a halogen in period 5.
Hence these elements consist of seven electrons in their outermost shell. There are a total of 14 metals in period 5 of the periodic table. The halogens are the elements in group 17 of the periodic table.
Select the element that is a halogen in Period 5. The electronic configuration of the halogen group is as shown below. The halogens are highly reactive nonmetallic elements.
Fluorine chemistry is the preserve of specialists because F_2 is extremely and dangerously reactive. For example rubidium has 5 electron shells a property found in all other period 5 elements whereas its electron configurations ending is similar to all other alkali metals. Fluorine F chlorine Cl bromine Br Iodine I astatine At.
Nitrous acid nitric acid e. Br Xe Te I N. It is a halogen in period 5.
MCQ Online Tests 30.

Periodic Table Of The Elements Halogens
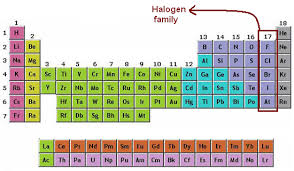
Halogens Fluorine Chlorine Bromine Iodine Astatine

Halogens On The Periodic Table Most Least Reactive Halogens Video Lesson Transcript Study Com

Comments
Post a Comment